Products
- Home
- / Products
- / MagLev Lab




MagLev Lab Device
$1,499.00
The VeriChem Lab MagLev DeviceTM revolutionizes the way forensic and chemistry labs approach powdered mixture separation. Engineered for efficiency, cost-effectiveness, and non-destructive analysis, this innovative tool uses magnetic levitation to separate water-soluble powdered mixtures based on the different density of the components.
Elevate your lab’s capabilities with the VeriChem Lab MagLev DeviceTM—where separation meets innovation.
Included:
- 1x MagLev Device
- 2 Reusable Containers (PMMA)
- Pelican Storage Case
- Separation Fluid for 10 Samples
Efficient Separation
MagLev separates components of powdered samples rapidly, saving valuable lab time and reducing bottlenecks in analysis workflows.
Non-Destructive Process
Preserves the integrity of the sample by separating components as powders, allowing for repeat analysis and further testing if needed.
Safety For Operators
Minimizes physical contact, creating a safer environment for lab technicians.
Enhanced Accuracy
Separates and concentrates components physically, reducing signal overlap or issues with detection thresholds using spectral methods.
Easy Integration
Compact and user-friendly, MagLev is designed to complement existing lab equipment and methods, providing a seamless addition to your workflow.
High-Precision Layering
Creates distinct, concentrated layers for each component, enhancing clarity in analysis and reducing cross-contamination for reliable, repeatable results.
For forensic applications, the MagLev device excels in isolating dilute components from complex mixtures, such as separating and concentrating dilute fentanyl (from 1% to 90%) from fentanyl-laced heroin—a critical capability in combating illicit drug challenges. It is equally useful in organic chemistry and pharmaceutical development, enabling the separation of crystals from different compounds or polymorphs.
Unlike traditional methods of separation, such as GC-MS or TLC, which require dissolving powders, the MagLev Device separates your samples in their powdered state through a completely non-destructive process. Say goodbye to labor-intensive techniques and embrace a cutting-edge solution that saves time and reduces costs with ease.
Below are examples of the different types of scheduled compounds that has been successfully separated using the MagLev device. The separation with MagLev, and the increase in concentration, enables accurate identification of scheduled drugs, adulterants, and diluents present in complex mixtures using secondary methods of analysis. Examples of such methods include, colorimetric reagent tests and Raman and FTIR-ATR spectrometers. MagLev can in most cases separate a powdered mixture as long as the powder is hydrophilic and does not dissolve in the hydrophobic separation liquid.
Scheduled compounds that MagLev has separated so far:
- Fentanyl·HCl
- Acetyl fentanyl·HCl
- Benzyl fentanyl·HCl
- Cocaine·HCl
- Heroin·HCl
- Methamphetamine·HCI
*Substances were obtained and separated in a DEA certified lab.
Device Specs
- Dimensions (LxWxH): 5″ by 5″ by 12″ (12.7cm by 12.7cm by 30cm)
- Weight: 2 lbs (0.9kg)
- Magnetic Field: 0.5 Tesla on the magnet surfaces that faces each other in the MagLev device.
- Materials: Rare-earth magnets in plastic holders that are fixed into position with stainless steel rods and hex nuts.
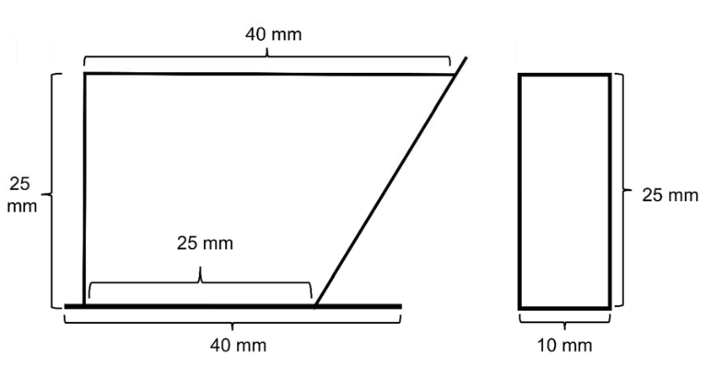
Separation Container Specs
- Dimensions: The Separation Container is 25 mm tall and it fits between the magnets of the MagLev device.
- Materials: PMMA plastic
Separation Liquid
- The Separation Liquid contains a patented Gadolinium compound mixed with hexane and tetrachloroethylene solvents.
- MSDS-Gadolinium compound
- MSDS-Hexane
- MSDS-Tetrachloroethylene
Accessories (not included)
- Operation: Pasteur pipettes for extraction of separated compounds.
- Cleaning: paper wipes, isopropanol or ethanol
- Waste Disposal: The Separation Liquid should be disposed of at a facility that accepts and takes care of hazardous chemical waste. Specifically, the waste should be handled as halogenated organic solvent waste. If the liquid waste also contains scheduled substances it should be disposed of according to regulations related to such substances.
Recommended PPE and Safety Precautions
- Nitrile Gloves. If the Separation Liquid spills on your gloves change to a new pair immediately.
- Lab Coat
- Safety Goggles
- The Separation Liquid should ideally be handled in a fume hood because it contains organic solvents (hexane and tetrachloroethylene) that gives of fumes should not be inhaled. The liquid can also be handled in a very well ventilated space or outside, but in such a scenario we strongly recommend the use of a respirator that can protect from the tetrachloroethylene, hexane, and any dust generation from the sampling of drugs. We recommend the 3M Disposable Respirator, Half Face Piece Assembly with Organic Vapor Respiratory Protection. A good cartridge that fit with this respirator is the 3M 6001 Organic Vapor Cartridge.
- The MagLev device contains magnets with strong magnetic fields. These magnets can attract objects made of certain metals, such as steel or iron. Keep such metal objects away from the MagLev device as fingers or skin can be pinched between the magnets and the attracted objects, posing a small to medium pinching hazard risk. The attracted objects could also damage the MagLev device.
- We recommend handling the Maglev device on a surface made of stone, wood, plastic or ceramic. Metal surfaces could be strongly attracted to the MagLev device, posing a small to medium pinching hazard risk.
- The magnetic field of the MagLev device could damage electronic equipment and digital storage devices, such as computers, mobile phones, and credit cards. Keep such items at least a 1-2 feet away from the MagLev device to avoid damage.
- The MagLev device is built to be rugged. However, if it breaks and the magnets come lose due to being dropped accidentally or similar, the two strong magnets of the device could be attracted to each other and pose a SEVERE pinching hazard risk. If this happens do not handle the loose magnets with your hands. Instead, handle the magnets with two non-metal sticks, such a wooden sticks. Use the sticks to place the magnets in the middle of a cardboard box packed with non-magnetic materials (1 foot of material all around the magnet). Examples of good non-magnetic materials include cardboard or paper. Dispose of the box with the magnets at a hazardous waste facility. Also, wear eye protection when handling the loose magnets as they can fly towards each other or be attracted to metal objects with high speed. The high-speed can result in the magnets breaking and sending potentially damaging shards in different directions.
Safety Precautions:
- Always wear PPE.
- Do not leave electronic equipment such as cell phones or objects that may be impacted by the strong magnets in the MagLev.
- Use nitrile gloves to prevent skin contact with the Separation Liquid. Change gloves the immediately if Separation liquid spills on them.
- The Separation Liquid should ideally be handled in a fume hood because it contains organic solvents (hexane and tetrachloroethylene) that gives of fumes should not be inhaled.
- The liquid can also be handled in a very well ventilated space or outside, but in such a scenario we strongly recommend the use of a respirator that can protect from the tetrachloroethylene, hexane, and any dust generation from the sampling of drugs.
- We recommend the 3M Disposable Respirator, Half Facepiece Assembly with Organic Vapor Respiratory Protection. A good cartridge that fit this respirator is the 3M 6001 Organic Vapor Cartridge.
Preparation of the area and device:
- Ensure the workspace is clean, dry, and free of obstructions.
- Verify the integrity and cleanliness of the container.
Preparing the container and adding the sample:
- Fill up the Separation container with Separation Liquid leaving 2-3 mm of headspace from the top edge (approximately 2.5 ml).
- Add up to 50 mg of the powdered sample to the filled container.
- Stir the powder sample in the Separation Liquid with a metal spoon or spatula for 5 seconds to disperse the powder.
Running the Separation:
- Place the filled container between the magnets.
- Let the separation proceed for until levitating clouds of separated compounds form. The separation typically takes 1-30 minutes, depending on the particle size of the powder. Smaller particles takes longer time to separate.
Extraction and Analysis
Extraction:
- Once the separation in the MagLev appears satisfactory, use a Pasteur pipette to extract each individual fraction separately.
- Collect fractions on filter paper and drop a few drops of hexane on the sample to remove (wick away) any residual Separation Liquid.
- Leave the sample for 2-3 minutes on the filter paper to allow the hexane to evaporate.
- The sample can now be analyzed with your analytical method of choice, such as a colorimetric reagent test or a FTIR-ATR spectrometer.
Analysis with portable Raman/FTIR:
- Once the separation has occurred, slowly slide the container so that the levitating clouds touch the sides of the container while the container remains between the magnets.
- When the clouds touch the container side, scan the clouds with a portable Raman spectrometer to identify the compound.
Disposal
- The Separation Liquid should be disposed of at a facility that accepts and takes care of hazardous chemical waste. Specifically, the waste should be handled as halogenated organic solvent waste.
- If the liquid waste also contains scheduled substances it should be disposed of according to regulations related to such substances.
- Dispose used pipettes as chemically contaminated sharp hazardous waste.
Cleaning:
- Rinse the container thoroughly with hexane 2-3 times and let it air dry.
- If the separation fluid has been spilled on the MagLev device, wipe it away with a tissue paper moistened with a little bit of hexane.
1. What is the MagLev Sample Preparation Device?
The MagLev is an advanced tool for preparing powdered samples in laboratory settings. Using Magnetic Levitation, it separates and concentrates multiple components in a non-destructive way, creating distinct layers that facilitate easy extraction and in-depth analysis.
2. How does the MagLev device work?
The device uses Magnetic Levitation to separate compounds based on their density forming levitating ‘clouds’ of the separated components without altering (dissolving) the sample. The clouds can be extracted using a Pasteur pipette and analyzed further with other methods of analysis. The isolated compound will typically be significantly higher in concentration compared to in the original sample mixture.
3. What types of samples can be used with MagLev?
The VeriChem MagLev is specifically designed for separation of powdered samples of water soluble compounds. Most illicit drugs are water soluble, but there are exceptions. The VeriChem MagLev can also seprate powdered mixtures om compounds that are hydrophobic. However, if separation of hydrophobic compounds, please contact us before you order from our website.
4. Is the process non-destructive?
Yes, MagLev’s levitation process is entirely non-destructive, preserving each compound in its original state. This allows for repeat analysis and further testing without consuming, altering, or damaging the sample.
5. How many components can MagLev separate?
MagLev can separate complex mixtures with multiple components, creating distinct layers for each. The exact number of separable components depends on the composition and properties of the sample. We have separated up to seven compounds at the same time, however, this number is not the upper limit.
6. What are the primary benefits of using MagLev?
MagLev offers cost-effective, fast, and non-destructive separation of powdered complex mixtures. Our customers tells us that they like that the MagLev can separate and concentrate the scheduled drugs above the limit of detection (typically 10-60%) for colorimetric reagent tests and Raman and FTIR-ATR spectrometers. The MagLev can also remove components that interfere with the analysis. Examples include compounds that form multiple different colors when the Marquis test is used, or fluorescent components that can interfere with spectroscopic analysis with Raman and FTIR-ATR.
7. Can MagLev be integrated into existing lab workflows?
Yes, MagLev’s compact design and straightforward operation make it easy to incorporate into any laboratory setting, complementing current sample preparation processes and equipment.
8. Does MagLev require any special training to operate?
Very litttle, MagLev is user-friendly and requires minimal training to use effectively. Standard operating procedures are provided to guide users through setup, operation, and cleanup.
9. How does MagLev improve lab workflow efficiency?
Fast separation (1-20 minutes) with MagLev could improve the initial screening of your samples by separating and concentrating the scheduled compounds. The concentrated compounds can in most cases be easily detected using colorimetric reagents and Raman and FTIR-ATR spectrometers. By knowing roughly which scheduled compounds are present before you run GC-MS you can in some cases shorten the run time and stream line process of derivation and selection of standards.
10. Is MagLev suitable for field testing?
Currently, MagLev is optimized for lab use where it can deliver highly accurate and reliable separation results. It can be used in the field, however, before you do we recommend that you talk to us for some guidance to ensure success.

Separation Fluid
$180.00 for 12 separations
VeriChem’s patented paramagnetic fluid for 12 MagLev separations.
Each box contains premeasured separation fluid in individual bottles for simple setup, usage and storage.
VeriChem can also customize your separation fluid based on the types of powders and substances you are interested in. Contact us to learn more.




